This site is intended for US Healthcare Professionals only
Start your patients on the only non-opioid intrathecal option
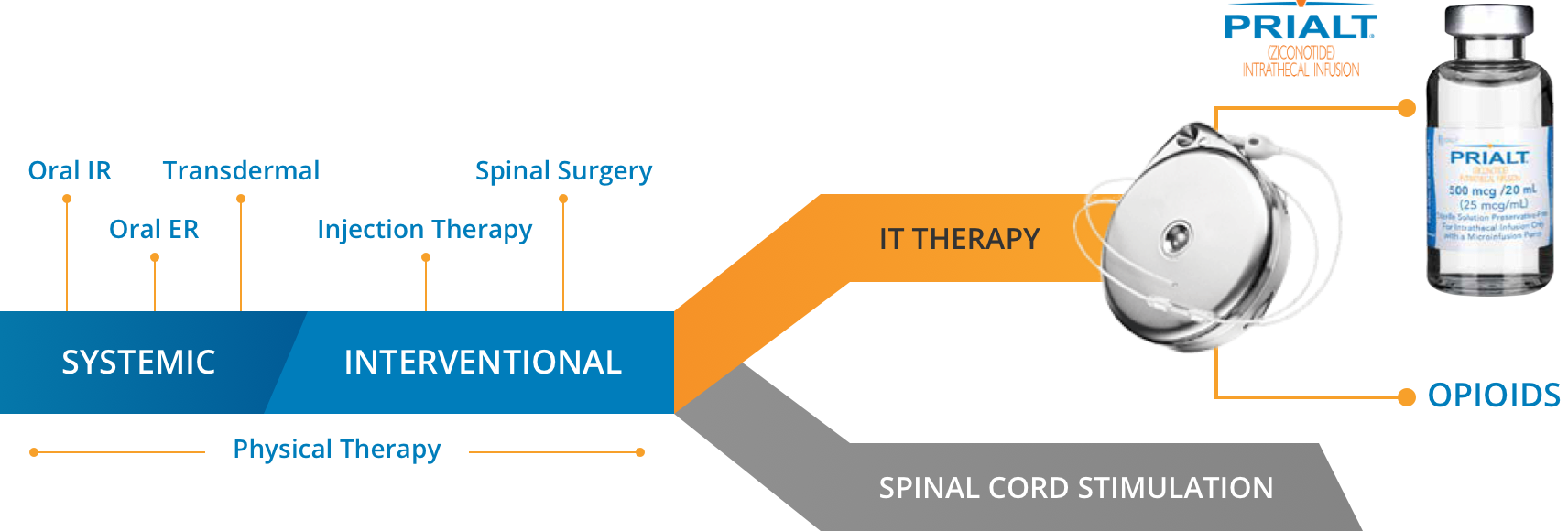
When to prescribe PRIALT, the only FDA-approved non-opioid intrathecal treatment for severe chronic pain
According to the 2016 Polyanalgesic Consensus Conference (PACC) guidelines, intrathecal therapy now occupies the same line as spinal cord stimulation in the management of severe chronic pain, with important caveats regarding your patient’s condition1:
- Before neurostimulation in cases of active cancer-related pain that is mechanical and likely to spread
- At the same time as neurostimulation in adults with non-cancer pain
- After neurostimulation for non-cancer pain if the pain is isolated and unlikely to spread1
PRIALT is recommended first-in-pump for cancer and non-cancer patients by the 2016 PACC guidelines.1
Steps for starting your patient on PRIALT
Establish patient expectations of intrathecal therapy:
- Explain that PRIALT is FDA-approved and PACC guideline-recommended for adults with severe chronic pain1,2
- Discuss the goals of treatment and that it may take time to respond2
- Educate patients on potential adverse reactions and monitor their response to treatment2
- Recommend psychological evaluation of patients to monitor for issues1
- Continually monitor their progress and assess their response to treatment, including improvements to pain severity2
Titration: start low and go slow
Studies show that tolerability improved when the patient’s PRIALT dose was started low and slowly titrated upward. Rapid titration schedules resulted in less tolerability and substantially more frequent adverse events. Slower titration may result in fewer serious adverse events and discontinuations for adverse reactions.1,2


Initiate1,4
A starting dose for continuous intrathecal delivery of 0.5-1.2 mcg/day—consistent with the PRIALT Prescribing Information—is recommended in the PACC guidelines,*† but initial dosing should not exceed 2.4 mcg/day (0.1 mcg/hour).
Titrate2
Titrate gradually, by no more than 2.4 mcg/day and no more than 2 to 3 times per week. Lower and less frequent dose increases may be used. The daily dose should not exceed 19.2 mcg/day.1,2,3
Monitor2
Ongoing assessment is key to managing outcomes. Adjust the dosage according to your patient’s pain severity, response to the therapy, and the occurrence of potential adverse events. To help manage expectations, let patients know that reaching the appropriate therapeutic dose may take longer when starting low.2
*PACC, founded 2000, offers guidance on IT therapy to help improve efficacy and patient safety. PACC was initiated by the International Neuromodulation Society (INS) and was partially funded via an unrestricted grant from product sponsor. No corporate entities had any direct input into the contents of the manuscripts or the conclusions of the collaborators.1
†PRIALT is intended for IT delivery using the Medtronic SynchroMed™ II infusion system and CADD-Micro® Ambulatory Infusion Pump. The minimum flow rate of the Medtronic SynchroMed™ II pump is 0.048 mL/day. The lowest initial starting dose that can be delivered through the Medtronic SynchroMed® II pump without dilution is 1.2 mcg/day.2,4
CADD-Micro® is a registered trademark of SIMS Deltec, Inc.
SynchroMed™ is a trademark of Medtronic
REFERENCES:
- Deer TR, Pope JE, Hayek SM, et al. The Polyanalgesic Consensus Conference (PACC): Recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation. 2017;20(2):3-31.
- PRIALT [package insert]. Lake Forest, IL; TerSera Therapeutics.
- Rauck RL, Wallace MS, Leong MS, et al. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31(5):403-404.
- SynchroMed II Drug Infusion Pump. Medtronic website. Accessed July 9, 2018. https://www.medtronic.com/us-en/healthcare-professionals/products/neurological/drug-infusion-systems/synchromed-ii.html
Support for you and your patients
Learn about our assistance program and download PA forms