Actor portrayals.
Start your patients on the only non‑opioid, intrathecal option
Efficacy and dosing
INDICATION
PRIALT® (ziconotide) solution, intrathecal infusion is indicated for the management of severe chronic pain in adult patients for whom intrathecal (IT) therapy is warranted, and who are intolerant of or refractory to other treatment, such as systemic analgesics, adjunctive therapies, or IT morphine.
Clinical studies and safety
The efficacy of intrathecal PRIALT in the management of severe chronic pain was studied in three double-blind, placebo-controlled, multicenter studies in a total of 457 patients (268 PRIALT, 189 placebo). The first two studies utilized a fast titration schedule that resulted in less tolerability and substantially more frequent adverse events. Therefore, the third study utilized a slow titration schedule as follows1:
- Randomized, double-blind, placebo-controlled study of PRIALT using the 21‑day slow titration schedule
- Conducted in adult patients with severe chronic pain not adequately controlled with intrathecal analgesics, including morphine, bupivacaine, and/or clonidine; or who were intolerant to analgesics and/or systemic analgesics
- Dosing with PRIALT was started at 2.4 mcg/day (0.1 mcg/hr)
- Dosing was increased by 2.4 mcg/day (0.1 mcg/hr) two to three times/week (minimum titration interval 24 hours) to a maximum dose of 19.2 mcg/day (0.8 mcg/hr) as needed for management of pain
- Final mean dose at the end of the trial at 21 days was 6.9 mcg/day (0.29 mcg/hr)
Slow titration, low-dose study results
In the third pivotal trial, PRIALT significantly reduced overall pain scores at Week 3 vs placebo (primary endpoint; p=0.04).1,2
The mean baseline pain scores in both the PRIALT and placebo groups were 81 mm on the VASPI (0-100 mm scale). After 3 weeks of treatment, the mean percent change in VASPI score from baseline improved by a mean of 12% in the PRIALT group vs 5% in the placebo group (intent-to-treat analysis; 95% [0.04%, 13%]).1,2

For adverse reactions in the slow titration, placebo-controlled trial, by percent, that occurred in ≥5% of patients and more commonly with PRIALT than with placebo, see Table 1 in Section 6.1 of the Prescribing Information.
The most frequently reported adverse reactions (≥25%) in clinical trials were dizziness, nausea, confusional state, and nystagmus.1
*Mean percent change in Visual Analog Score of Pain Intensity (VASPI) score from baseline to Day 21 for both groups.1,2
PRIZM registry3
The Patient Registry of Intrathecal Ziconotide Management (PRIZM) was a prospective, open-label, long-term, multicenter, observational study (registry) of intrathecal ziconotide in a real-world setting for adults with severe chronic pain.
- Objectives: Evaluate the short- and long-term safety and effectiveness of IT ziconotide based on patient-reported outcomes
- Primary efficacy outcome: Change in numeric pain rating scale (NPRS) score from baseline to Week 12 after enrollment
- NPRS scores were assessed at every clinic visit, including at 6, 9, 12, 15, and 18 months
The results of the monotherapy group are presented below:
- 93 patients enrolled in the study, 74 patients completed 12 weeks (primary endpoint), and 60, 46, 37, 30, and 29 patients remained in the registry at Months 6, 9, 12, 15, and 18 months
- 86% of patients were on ziconotide monotherapy at Week 12, 74% at Month 6, 55% at Month 12, and 57% at Month 18
- Mean reduction in pain intensity level (NPRS score) over baseline was greater in the monotherapy group and led to a greater percentage of patients with a clinically meaningful response (30% reduction in NPRS score) at all time points
- Week 12: 26%; Month 6: 41%; Month 9: 44%; Month 12: 50%; Month 15: 60%; Month 18: 43%
- Greater percentage of patients receiving combination therapy (82.8%) discontinued the study compared with those receiving monotherapy (64.1%)
Almost all patients experienced adverse events, the most common of which were nausea (25.8%), confusional state (22.6%), and dizziness (20.4%). The treatment-emergent adverse event profile was similar to what was observed in the clinical trial program.1
When to prescribe PRIALT – the only FDA-approved, non-opioid, intrathecal treatment for severe chronic pain
According to the 2016 Polyanalgesic Consensus Conference (PACC) guidelines, intrathecal therapy now occupies the same line as spinal cord stimulation in the management of severe chronic pain, with important caveats regarding your patient’s condition4:
- Before neurostimulation in cases of active cancer-related pain that is mechanical and likely to spread
- As an equal line of therapy with neurostimulation in adults with severe chronic, non-cancer pain
- After neurostimulation for non-cancer pain if the pain is isolated and unlikely to spread
PRIALT is recommended first-in-pump for cancer and non-cancer patients by the 2016 PACC guidelines.4
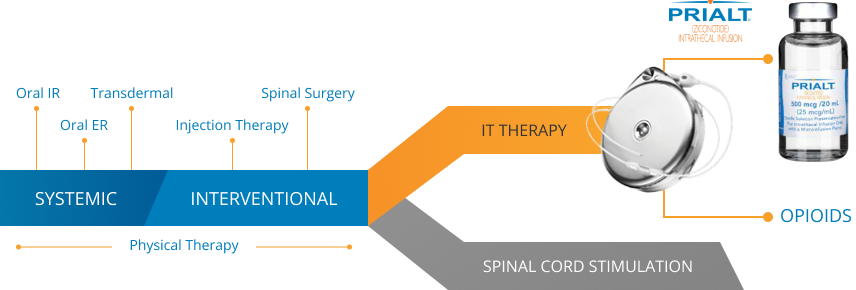

ER=extended release; IR=immediate release.
Steps for starting your patients on PRIALT
Establish patient expectations of intrathecal therapy:
- Explain that PRIALT is FDA-approved and PACC guideline-recommended for adults with severe chronic pain1,4
- Discuss the goals of treatment and that it may take time to respond1
- Educate patients on potential adverse reactions and monitor their response to treatment1
- Recommend psychological evaluation of patients to monitor for issues4
- Continually monitor patients’ progress and assess their response to treatment, including improvements to pain severity1
Dosing and titration: Start low and go slow
Studies show that tolerability improved when the patient’s PRIALT dose was started low and slowly titrated upward. Rapid titration schedules resulted in less tolerability and substantially more frequent adverse events. Slower titration may result in fewer serious adverse events and discontinuations for adverse reactions.1,4


Initiate1,4
A starting dose for continuous intrathecal delivery of 0.5-1.2 mcg/day – consistent with the PRIALT Prescribing Information – is recommended in the PACC guidelines,†‡ but initial dosing should not exceed 2.4 mcg/day (0.1 mcg/hour).
Titrate1
Titrate gradually, by no more than 2.4 mcg/day and no more than 2 to 3 times per week. Lower and less frequent dose increases may be used. The daily dose should not exceed 19.2 mcg/day.1,2,4
Monitor1
Ongoing assessment is key to managing outcomes. Adjust the dosage according to your patient’s pain severity, response to the therapy, and the occurrence of potential adverse events. To help manage expectations, let patients know that reaching the appropriate therapeutic dose may take longer when starting low.
†PACC, founded 2000, offers guidance on IT therapy to help improve efficacy and patient safety. PACC was initiated by the International Neuromodulation Society (INS) and was partially funded via an unrestricted grant from product sponsor. No corporate entities had any direct input into the contents of the manuscripts or the conclusions of the collaborators.4
‡PRIALT is intended for IT delivery using the Medtronic SynchroMed® II and Medtronic SynchroMed® III infusion systems and CADD‑Micro® Ambulatory Infusion Pump. The minimum flow rate of the Medtronic SynchroMed® II and Medtronic SynchroMed® III pump is 0.048 mL/day. The lowest initial starting dose that can be delivered through the Medtronic SynchroMed® II and Medtronic SynchroMed® III pump without dilution is 1.2 mcg/day.1,5
CADD-Micro® is a registered trademark of SIMS Deltec, Inc.
SynchroMed® is a registered trademark of Medtronic, Inc.
References: 1. PRIALT® (ziconotide). Prescribing Information. TerSera Therapeutics LLC. 2. Rauck RL, Wallace MS, Leong MS, et al. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31(5):393-406. 3. McDowell GC, Saulino MF, Wallace M, et al. Effectiveness and safety of intrathecal ziconotide: final results of the Patient Registry of Intrathecal Ziconotide Management (PRIZM). Pain Med. 2020;21(11):2925‑2938. 4. Deer TR, Pope JE, Hayek SM, et al. The Polyanalgesic Consensus Conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation. 2017;20(2):96-132. 5. SynchroMed II Drug Infusion Pump. Medtronic website. https://www.medtronic.com/us-en/healthcare-professionals/products/neurological/drug-infusion-systems/synchromed-ii.html. Updated July 2022. Accessed September 1, 2023.
SUPPORT FOR YOU AND YOUR PATIENTS
Learn about PRIALT coverage support and download resources